A27 - Synaptic scaffolds regulating cholinergic Drosophila synapses
Prinzipal Investigator
Prof. Dr. David Owald, Charité Berlin
The protein composition of synapses can remain stable or be plastically rearranged. Stability is valuable for relaying a specific content (such as the identity of sensory stimuli), plasticity for associating information during learning processes. At glutamatergic synapses, stable or plastic properties are controlled by multidomain scaffolding proteins that allow for the incorporation, exchange but also retention of postsynaptic receptor proteins. How individual scaffolding proteins define static or dynamic properties of specific synapse types is however largely unclear. Likewise, causally connecting molecular scaffold organization directly with synaptic function in brain circuits controlling an animal’s behavior remains challenging.
We recently discovered that both, synapses conveying stable sensory relay, but also synapses storing learned information, use fast cholinergic neurochemistry in the Drosophila brain. Moreover, our preliminary experiments show that short-term memory requires postsynaptic nicotinic acetylcholine receptors (Figure 1). This poses the hypothesis that postsynaptic cholinergic receptors might be dynamically regulated at plastic cholinergic synapses but kept more stable at relay-type synapses.
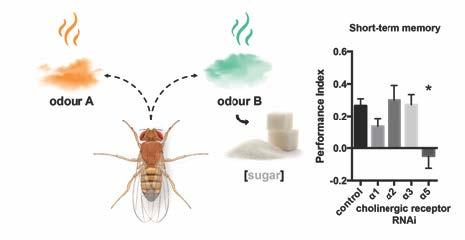
Figure 1: Specific postsynaptic nicotinic receptors are needed at specific plastic cholinergic synapses for learning and memory in Drosophila. Genetically-controlled cell-specific knockdown of a subset of essential cholinergic receptor α-subunits that together with other α and β subunits subtypes form cholinergic receptors abolishes appetitive short-term memory. Thus, memory storage requires plastic regulation of at least α-subunit 5. We so far tested 4 out of 10 subunits, all present at this synapse. Importantly, we find that RNAi knock-down only mildly affects synaptic transmission.
Our biochemical studies have identified a postsynaptic scaffold comprised of the postsynaptically enriched protein DREP2 that we show to interact with the 4.1 family member CDEP and the TIAM orthologous Rho/Rac guanine nucleotide exchange factor SIF1. We also identified the 4.1 protein Coracle to interact with nicotinic acetylcholine receptors and the multi-PDZ domain protein PSD-95 (Discs Large, Dlg) to be highly enriched at the postsynaptic densities of cholinergic synapses. Our data furthermore suggest that DREP2, CDEP and SIF1 are part of a common complex and we found CDEP and SIF1 to robustly localize to cholinergic postsynapses (Figure 2). Importantly, our preliminary data have linked a distinct scaffolding protein to the expression of plasticity-related behavioral output, suggesting that synapse type-specific individual ‘scaffold building blocks’ could shape static and plastic properties.
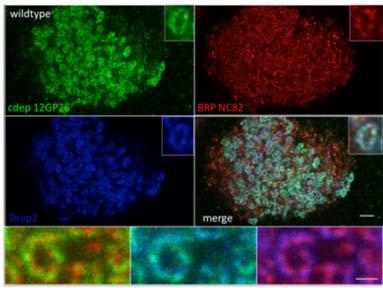
Figure 2: CDEP (green) and DREP2 (blue) colocalize to postsynaptic densities of type 1 cholinergic synapses (‘ring-like structures’) opposite to the presynaptic marker Nc82 (BRP).
Using targeted genetics in combination with co-immunoprecipitations, we are systematically analyzing the postsynaptic scaffold composition of distinct cholinergic synapses. We address how scaffolding proteins distribute to plastic and static cholinergic synapses and visualize their postsynaptic distribution using super-resolution microscopy. Mutual interactions are investigated by probing protein localization in combination with genetically targeted RNAi knockdown of the scaffolding proteins specific to the involved cell types. Functionally, we directly test whether scaffolding proteins regulate postsynaptic receptor dynamics by in vivo time-lapse imaging of the cholinergic α-subunit 7 receptor and test learned behavior following knock-down of selected scaffolding proteins at individual synapse types. Collectively, our studies will help to uncover how distinct receptor-associated proteinscaffolds define plastic versus static properties.
References:
- Ehmann, N., D. Owald, and R.J. Kittel. Drosophila active zones: From molecules to behaviour. Neurosci Res. 127: 14-24 (2018)
- Ehmann N, Owald D, Kittel RJ. Drosophila active zones: From molecules to behaviour. Neurosci Res. 127:14-24 (2018) (Review)
- Reddy-Alla, S., M.A. Böhme, E. Reynolds, C. Beis, A.T. Grasskamp M.M. Mampell, M. Maglione, M. Jusyte, U. Rey, H. Babikir, A.W. McCarthy, C. Quentin, T. Matkovic, D.D. Bergeron, Z. Mushtaq, F. Göttfert, D. Owald, T. Mielke, S.W. Hell, S.J. Sigrist, and A.M. Walter. Stable Positioning of Unc13 Restricts Synaptic Vesicle Fusion to Defined Release Sites to Promote Synchronous Neurotransmission. Neuron. 95: 1350-1364 (2017)
- Perisse, E., D. Owald, O. Barnstedt, C.B. Talbot, W. Huetteroth, and S. Waddell. Aversive learning and appetitive motivation toggle feed-forward inhibition in the Drosophila mushroom body. Neuron. 90: 1086-99 (2016)
- Barnstedt, O., D. Owald, J. Felsenberg, R. Brain, J.P. Moszynski, C.B. Talbot, P.N. Perrat, and S. Waddell. Memory-relevant mushroom body output synapses are cholinergic. Neuron. 89:1237-47 (2016)
- Owald, D., J. Felsenberg, C.B. Talbot, G. Das, E. Perisse, W. Huetteroth, and S. Waddell. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron. 86: 417-27 (2015)
- Owald D & Waddell S. Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila. Curr Opin Neurobiol. 35:178-84 (2015) (Review)
- Burke, C.J., W. Huetteroth, D. Owald, E. Perisse, M.J. Krashe, G. Das, D. Gohl, M. Silies, S. Certel, and S. Waddell. Layered reward signaling through octopamine and dopamine in Drosophila. Nature. 492: 433-7 (2012)
- Owald, D., O. Khorramshahi, V.K. Gupta, D. Banovic, H. Depner, W. Fouquet, C. Wichmann, S. Mertel, S. Eime, E. Reynolds, M. Holt, H. Aberle, and S.J. Sigrist. Cooperation of Syd-1 with Neurexin synchronizes pre- with postsynaptic assembly. Nature Neuroscience. 15: 1219-26 (2012)
- Banovic, D., O. Khorramshahi, D. Owald, C. Wichmann, T. Riedt, R. Tian, S.J. Sigrist, and H. Aberle. Drosophila Neuroligin 1 coordinates pre- and postsynaptic assembly. Neuron. 66: 724-38 (2010)
- Owald, D., W. Fouquet, M. Schmidt, C. Wichmann, S. Mertel, H. Depner, F. Christiansen, C. Zube, C. Quentin, J. Korner, H. Urlaub, K. Mechtler, and S.J. Sigrist. A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila. J Cell Biol. 188: 565-79 (2010)
- Fouquet, W., D. Owald, C. Wichmann, S. Mertel, H. Depner, M. Dyba, S. Hallermann, R.J. Kittel, S. Eimer, and S.J. Sigrist. Maturation of active zone assembly by Drosophila Bruchpilot. J Cell Biol. 186, 129-45 (2009)