A22 - Investigating the role of the membrane-associated Cerebral Cavernous Malformations (CCM) protein scaffold in biomechanical signaling
Principal Investigator
Prof. Dr. Salim Seyfried, Universität Potsdam
Many cell types and tissues are affected by mechanical stimuli that bias their development and physiology. For instance, morphogenesis and maintenance of blood vessels is critically dependent on mechanical stimuli due to hemodynamic forces. We found that a loss of proteins of the membrane-associated Cerebral Cavernous Malformation (CCM) protein scaffold in zebrafish causes an elevated expression of the mechanosensitive gene krüppel-like factor 2a (klf2a). Three proteins of the CCM scaffold (CCM1, CCM2, and CCM3) assemble around the transmembrane protein Heart development protein with EGF like domains 1 (HEG1). In turn, the CCM protein complex is involved in endothelial junctional stabilization by directly interacting with the vascular endothelial- cadherin (VE-Cadherin) complex (Fig. 1).
A loss of CCM proteins causes a number of cardiovascular malformations in zebrafish including cardiac ballooning, heart looping defects, a failure of endocardial cushions to form, and defective blood vessel formation (Fig. 2). In functional studies, we found that strong upregulation of klf2a expression within the zebrafish vasculature causes phenotypes that resemble the loss of CCM scaffold proteins. Hence, the effects due to a loss of CCM proteins can be attributed to an elevated biomechanical signaling output within endothelial cells. We discovered that levels of Cerebral Cavernous Malformations (CCM) scaffold proteins are regulated by hemodynamic forces. We also found that the overexpression of CCM scaffold proteins downregulates the expression of Klf2a. We generated stable transgenic lines of zebrafish expressing CCM protein fusions with EGFP or mCherry and characterized their subcellular localization patterns within the developing vasculature of zebrafish. This work revealed that the CCM proteins correctly localize in the absence of VE-cadherin. A pharmacological suppression screen in Ccm2-deficient zebrafish embryos provided important new insights into relevant molecular players that interact with the CCM scaffold.
Project A22 aims at elucidating the precise dynamics of CCM scaffold assembly and at identifying associated proteins during zebrafish endothelial development. In functional studies, using appropriate zebrafish mutants, we will address how the loss of AJs components impacts CCM protein scaffold assembly. Conversely, we will study the role of the CCM protein scaffold for AJs maturation within nascent blood vessels. Transgenic reporter lines for AJs are available for these analyses. In part, these studies will involve functional developmental genetics and cell biological studies. We will also put a strong emphasis on developing machine learning algorithms for advanced image analysis and work on improving superresolution microscopy techniques for in vivo applications in the zebrafish embryo.
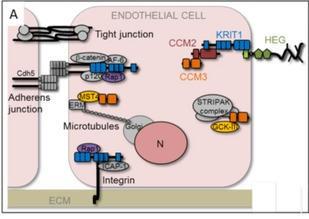
Figure 1: Crosstalk of membrane-associated junctional complexes and signaling pathways with the CCM protein scaffold. The membrane-associated Heart of glass (HEG) protein scaffold, VE-cadherin (Cdh5)-based endothelial AJs, and integrin-based cell-extracellular matrix junctions play important roles in different steps of vessel morphogenesis. The two junctional complexes directly interact with the CCM protein scaffold via KRIT1/CCM1 or ICAP1. Direct interactions of KRIT1 and CCM3 with other proteins are indicated. CCM3 also interacts with kinases of the germinal center kinase III (GCK-III) family and with the striatin-interacting phosphatase and kinase (STRIPAK) complex (modified from 1).
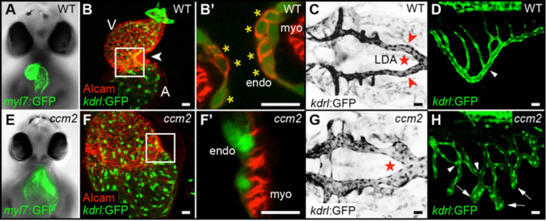
Figure 2: Cardiovascular defects in the zebrafish embryo associated with the loss of CCM2. (A-D) Wild-type and (E-H) ccm2m201 mutant embryos. (A,E) Frontal views of 48 hours post fertilization (hpf) hearts of the myocardial reporter line Tg(myl7:GFP)twu34. (E) Cardiac expansion phenotype in ccm2m201 mutant. (B,F) Cardiac malformation phenotype in ccm2m201 mutants. (B’,F’) Shown are hearts of different genotypes at 48 hpf with a single confocal plane section at the atrioventricular canal region. Endocardial cushion cells are marked by Tg(kdrl:GFP)s843 and Alcam staining (asterisks). (F’) Complete lack of cardiac cushions in ccm2m201 mutant. (C,G) Dorsal views of the lateral dorsal aorta (LDA) at 48 hpf. The lateral dorsal aorta morphology is marked by Tg(kdrl:GFP)s843 expression (inverted image). (G) Overgrowth of the ccm2m201mutant lateral dorsal aorta. (D,H) Ectopic sprouts and vessel branch point defects within the subintestinal vein in ccm2m201mutants. A, atrium; V, ventricle
References:
- Otten C, Knox J, Boulday G, Eymery M, Haniszewski M, Neuenschwander M, Radetzki S, Vogt I, Hähn K, De Luca C, Cardoso C, Hamad S, Igual Gil C, Roy P, Albiges-Rizo C, Faurobert E, von Kries JP, Campillos M, Tournier-Lasserve E, Derry WB, Abdelilah-Seyfried S. Systematic pharmacological screens uncover novel pathways involved in cerebral cavernous malformations. EMBO Mol Med. 10(10):e9155 (2018)
- Donat S, Lourenço M, Paolini A, Otten C, Renz M, Abdelilah-Seyfried S. Heg1 and Ccm1/2 proteins control endocardial mechanosensitivity during zebrafish valvulogenesis. Elife. 7:e28939 (2018)
- Lisowska J, Rödel CJ, Manet S, Miroshnikova YA, Boyault C, Planus E, De Mets R, Lee HH, Destaing O, Mertani H, Boulday G, Tournier-Lasserve E, Balland M, Abdelilah-Seyfried S, Albiges-Rizo C, Faurobert E. The CCM1-CCM2 complex controls complementary functions of ROCK1 and ROCK2 that are required for endothelial integrity. J Cell Sci. 131(15):jcs216093 (2018)
- Paolini A, Abdelilah-Seyfried S. The mechanobiology of zebrafish cardiac valve leaflet formation. Curr Opin Cell Biol. 55:52-58 (2018) (Review)
- Haack T, Abdelilah-Seyfried S. The force within: endocardial development, mechanotransduction and signalling during cardiac morphogenesis. Development. 143(3):373-86 (2016) (Review)
- Renz M, Otten C, Faurobert E, Rudolph F, Zhu Y, Boulday G, Duchene J, Mickoleit M, Dietrich AC, Ramspacher C, Steed E, Manet-Dupé S, Benz A, Hassel D, Vermot J, Huisken J, Tournier-Lasserve E, Felbor U, Sure U, Albiges-Rizo C, Abdelilah-Seyfried S. Regulation of β1 integrin-Klf2-mediated angiogenesis by CCM proteins. Dev Cell. 32(2):181-90 (2015)
- Dietrich AC, Lombardo VA, Veerkamp J, Priller F, Abdelilah-Seyfried S. Blood flow and Bmp signaling control endocardial chamber morphogenesis. Dev Cell. 30(4):367-77 (2014)
- Veerkamp J, Rudolph F, Cseresnyes Z, Priller F, Otten C, Renz M, Schaefer L, Abdelilah-Seyfried S. Unilateral dampening of Bmp activity by nodal generates cardiac left-right asymmetry. Dev Cell. 24(6):660-7 (2013)
- Zhang J, Piontek J, Wolburg H, Piehl C, Liss M, Otten C, Christ A, Willnow TE, Blasig IE, Abdelilah-Seyfried S. Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion. Proc Natl Acad Sci U S A. 107(4):1425-30 (2010)
- Rohr S, Otten C, Abdelilah-Seyfried S. Asymmetric involution of the myocardial field drives heart tube formation in zebrafish. Circ Res. 102(2):e12-9 (2008)