A16 - A PRG2-PTEN based membrane scaffold involved in the formation of axon branches
Principal Investigator:
Prof. Dr. Britta Eickholt, Charité
The PTEN phosphatase represents a key node in protein-protein interaction networks and, through binding and modulation of other signaling components, regulates diverse aspects of cellular morphology and homeostasis (Cosker & Eickholt, 2007; Song et al., 2012). PTEN functions predominately at the cell membrane, converting phosphatidylinositol 3,4,5-trisphosphate (PIP3) to phosphatidylinositol 4,5-bisphosphate (PI4,5P2), thereby directly antagonizing the activity of phosphoinositide 3-kinase (PI3K) and its established downstream signaling pathways. Much progress has been made in characterizing the function of PTEN and its binding partners in neurons, especially during the processes of neurite outgrowth and synapse formation (Wichterle et al., 2002; van Diepen & Eickholt, 2008; Waite & Eickholt, 2010). However, how such PTEN-based scaffolds are dynamically regulated and involved in regulating PI3K or other signaling systems is less clear.
We propose, here, to explore the role of membranous PTEN scaffolds in the vicinity of the plasma membrane, which regulate cortical actin dynamics and/or membrane trafficking during neuronal circuit formation. Our previous proteomics screen of components of PTEN-based scaffolds in neurons identified several cytosolic PTEN-interacting proteins (for example members of the Myosin V family of motorproteins and the actin-binding protein Drebrin (DBN) (van Diepen et al., 2009; Kreis et al., 2013), as well as membrane associated proteins (for example members of a family of transmembrane Lipid Phosphatases).
To analyse the function of PTEN scaffolds we will use neurons derived directly from embryonic stem cells (ES cells) as our primary experimental system (Figure 1). ES cell-derived neurons allow us to rapidly introduce genetic modifications such as fluorescent fusion proteins, cell compartment-specific variants, null mutations, and RNAi knock-downs and then to systematically assay the interaction of PTEN pathway components through epistasis experiments.
Finally, we will apply SILAC-based quantitative proteomic technologies in ES cells derived neurons in order to probe for the existence of other distinct neuronal PTEN scaffolds that associate in specific cellular compartments.
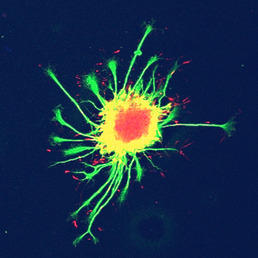
Figure 1: ES cell-derived motor neurons - cultured here as embryoid bodies - produce extensive
networks of axons. Axon extension is facilitated by growth cones, which resemble
closely the morphology of primary neurons cultured in vitro.
References
- Kreis P, Hendricusdottir R, Kay L, Papageorgiou IE, van Diepen M, Mack T, Ryves J, Harwood A, Leslie NR, Kann O, Parsons M, Eickholt BJ. Phosphorylation of the Actin Binding Protein Drebrin at S647 Is Regulated by Neuronal Activity and PTEN. PLoS One 8, e71957 (2013)
- Song, M.S., Salmena, L. & Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13, 283-296 (2012) (Review)
- Waite, K. & Eickholt, B.J. The Neurodevelopmental Implications of PI3K Signaling. Curr Top Microbiol Immunol (2010)
- van Diepen MT, Parsons M, Downes CP, Leslie NR, Hindges R, Eickholt BJ. MyosinV controls PTEN function and neuronal cell size. Nat Cell Biol 11, 1191-1196 (2009)
- van Diepen, M.T. & Eickholt, B.J. Function of PTEN during the formation and maintenance of neuronal circuits in the brain. Dev Neurosci 30, 59-64 (2008)
- Cosker, K.E. & Eickholt, B.J. Phosphoinositide 3-kinase signalling events controlling axonal morphogenesis. Biochem Soc Trans 35, 207-210 (2007) (Review)
- Wichterle, H., Lieberam, I., Porter, J.A. & Jessell, T.M. Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385-397 (2002)