A13 - Role of the GTPase ARFRP1 in the organization of Golgi-associated protein scaffolds that regulate lipid droplets biogenesis and turnover
Principal Investigator
Prof. Dr. Annette Schümann, DIfE
Cytosolic lipids are surrounded by a phospholipid monolayer and associating proteins that influence lipid droplet growth and degradation. We aim to study specific protein and vesicular trafficking processes that are required for lipid droplet turnover.
We have previously shown that the GTPase ARFRP1 modifies lipid droplet formation by two mechanisms inhibiting lipolysis and regulating lipid droplet fusion (Fig. 1). We therefore hypothesize that ARFRP1 regulates the organization of membrane-associated multidomain scaffolding proteins required for lipid droplet turnover. We will focus (1) on scaffolds assembled at the Golgi in response to ARFRP1 action (like golgin proteins), which participate in lipid droplet formation, and (2) will study the role of ARFRP1 for the localization of lipid droplet coating proteins (PAT proteins) in respect to the regulation of lipolysis. With cell and animal models lacking the ARFRP1 we will perform biochemical and cell biological studies including several microscopic techniques, pulldown and co-immunoprecipiÂtation experiments in order to understand how membrane scaffolds at the Golgi contribute to the turnover of lipid droplets. Specifically we will
- identify ARFRP1-specific guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs) and GTP-dependent effectors in order to understand the regulation of ARFRP1 activity and identify upstream signalling pathways and downstream target
- test whether ARFRP1 regulates lipid droplet size by
- inducing the ARL1-golgin-Rab cascade (Fig. 2), thereby modulating vesicular transport required for lipid droplet formation and function,
- mediating the targeting of PAT proteins to the lipid droplet,
- controlling the targeting of regulatory proteins such as lipases and their binding partners (e.g. CGI-58) to the lipid droplet surface.
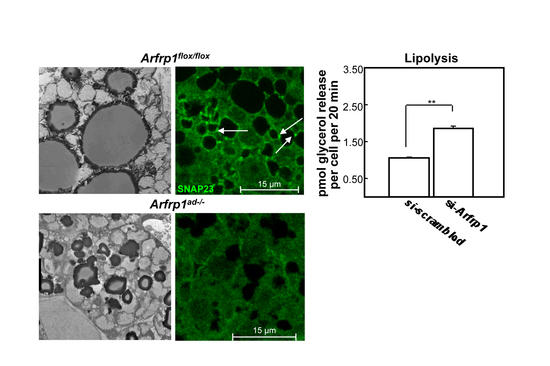
Fig. 1: Reduced size of lipid droplets in adipose tissue of mice lacking Arfrp1(Arfrp1ad-/-) and impaired SNAP23-mediated fusion of lipid droplets. Increased lipolysis in 3T3-L1 adipocytes after suppression of Arfrp1expression.
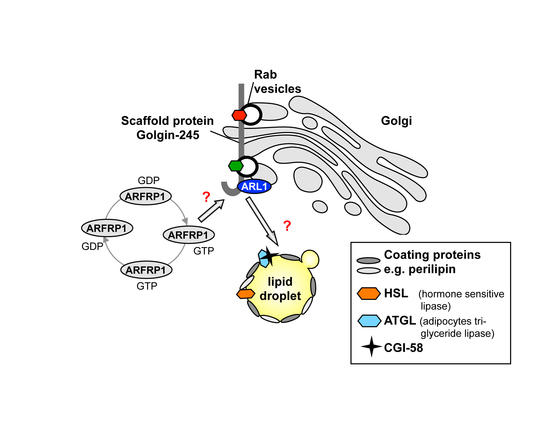
Fig. 2: Proposed role of ARFRP1 for recruitment of the scaffold protein Golgin-245 to the Golgi and the subsequent targeting of proteins to the lipid droplet and induction of lipid droplet fusion.
Publications
1. Zahn, C., Hommel, A., Lu, L., Hong, W., Walther, D.J., Florian, S., Joost, H.-G., Schürmann, A. (2006) Knockout of Arfrp1 leads to disruption of ARF-like1 (ARL1) targeting to the trans-Golgi in mouse embryos and HeLa cells . Mol Membr Biol. 23, 475-485
2. Zahn, C., Jaschke, A., Weiske, J., Hommel, A., Hesse, D., Augustin, R., Lu, L., Hong, W., Florian, S., Scheepers, A., Joost, H.G., Huber, O., Schürmann A. (2008) ADP-ribosylation factor-like GTPase ARFRP1 is required for trans-Golgi to plasma membrane trafficking of E-cadherin. J. Biol. Chem. 283, 27179-27188
3. Hommel, A., Hesse, D., Völker, W., Jaschke, A., Moser, M., Engel, T., Blüher, M., Zahn, C., Chadt, A., Ruschke, K., Vogel, H., Kluge, R., Robenek, H., Joost, H.G., Schürmann, A. (2010) The ARF-Like GTPase ARFRP1 Is Essential for Lipid Droplet Growth and Is Involved in the Regulation of Lipolysis. Mol. Cell. Biol. 30, 1231-1242
4. Hesse, D., Hommel, A., Jaschke, A., Moser, M., Bernhardt, U., Zahn, C., Kluge, R., Wittschen, P., Gruber, A.D., Al-Hasani, H., Joost, H.G., Schürmann, A. (2010) Altered GLUT4 trafficking in adipocytes in the absence of the GTPase Arfrp1. Biochem. Biophys. Res. Commun. 394, 896-903
5. Jaschke, A., Chung, B., Hesse, D., Kluge, R., Zahn, C., Moser, M., Petzke, K.J., Brigelius-Flohé, R., Puchkov, D., Koepsell, H., Heeren, J., Joost, H.G., Schürmann, A. (2012) The GTPase ARFRP1 controls the lipidation of chylomicrons in the Golgi of the intestinal epithelium. Hum. Mol. Genet. [Epub ahead of print] PubMed
