A01 - Structural and functional organization of endocytic scaffolds within the periactive zone
Principal Investigators:
Prof. Dr. Volker Haucke, Freie Universität Berlin
Dr. Tanja Maritzen, Freie Universität Berlin
Communication within the nervous system involves the exocytic fusion of neurotransmitter-filled synaptic vesicles (SVs), neurosecreÂtory organelles that comprise distinct sets of proteins and lipids, at active zone membranes. Following exocytic fusion SVs are recycled locally (1) (Figure 1) largely via clathrin-mediated endocytosis (CME) of SV proteins within the surrounding periactive zone. Clathrin-based SV recycling in addition to clathrin (2) involves endocytic adaptors such as stonin 2 (3) and AP180 (4) as well as accessory proteins that contribute to SV cargo sorting, membrane deformation, remodelling, and fission. Although subÂstantial progress has been made towards unravelling the mechanics of CME, the spatio-temporal dynamics of endocytic proteins within the endocytic or periactive zone and the underlying molecular mechanisms have remained elusive. Such spatio-temporal control of the SV cycle within the nerve terminal likely involves membrane-associated multidomain scaffolds including intersectin (5) (Figure 2) and Eps15.
Within project A01 we investigate the role of Eps15/intersectin as a molecular scaffold linking SV sorting adaptors such as stonin 2/stoned B to periactive zone organization. We make combined use of cell biologiÂcal, biochemical, and genetic approaches, paired with electron and super-resolution light microÂscopy (SRLM) techniques to unravel the structural and functional organization of the Eps15/ interÂsectin-based endocytic scaffold at synapses. On the long run we expect to gain new insights into the role of endocytic scaffolds in presynaptic organization and exo-endocytic coupling.
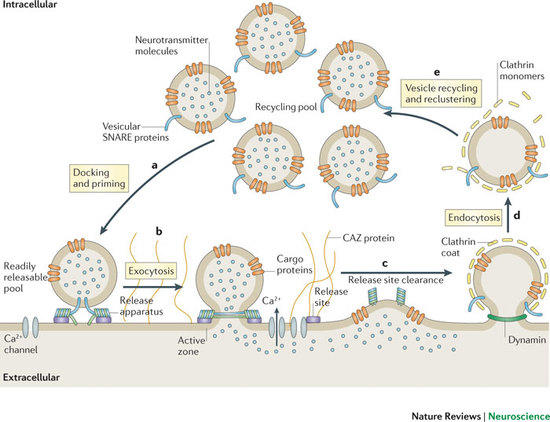
Fig. 1: Schematic depiction of the exo-endocytic cycle of SVs. The AZ is a specialized matrix for exocytosis, whereas SV endocytoÂsis preferentially occurs at the surÂrounding periactive zone. SV proÂteins other than synaptobrevin are summarized as "cargo proteins" (taken from Haucke et al., Nat Rev Neurosci 2011).
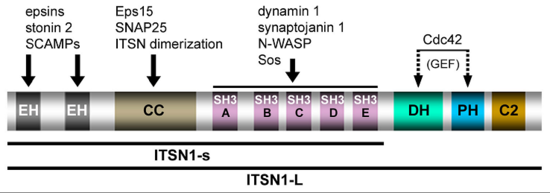
Fig. 2: Domain structure and protein interactions of intersectin 1L (ITSN1-L) (taken from Pechstein et al., Biochem. Soc. Trans. 2010).
References:
1. Haucke, V., Neher, E., Sigrist, S.J. (2011) Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci. 12, 125-136
2. von Kleist, L. et al (2011) Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell, 146, 471-484
3. Diril, M. K., Wienisch, M., Jung, N., Klingauf, J., and Haucke, V. (2006) Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization. Dev. Cell 10, 233-244
4. Koo, S.Y., Markovic, S., Puchkov, D., Mahrenholz, C., Beceren-Braun, F., Maritzen, T., Dernedde, J., Volkmer, R., Oschkinat, O., Haucke, V. (2011) SNARE motif-mediated sorting of synaptobrevin by the endocytic adaptors CALM and AP180 at synapses. Proc. Natl. Acad. Sci. USA, 108, [advance online publication]
5. Pechstein, A., Bacetic, J., Vahedi-Faridi, A., Gromova, K., Sundborger, A., Tomlin, N., Krainer, N., Vorontsova, O., Schäfer, J.G., Owe, S.G., Cousin, M.A., Saenger, W., Shupliakov, O., and Haucke, V. (2010) Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2. Proc. Natl. Acad. Sci. USA, 107, 4206-4211
