A12 - Structural and functional examinations of nucleotide-dependent membrane scaffolds
Principal Investigator
Eps15 homology (EH) domain containing proteins (EHDs) are ubiquitously expressed dynamin-related ATPases which regulate membrane trafficking pathways originating from the plasma membrane and from internal membrane compartments. Similar to other dynamin superfamily members, EHDs assemble on membranes to form ATP-dependent oligomeric scaffolds that remodel the underlying membrane. We previously solved the crystal structure of dimeric EHD2 in its auto-inhibited form, comprising a GTPase domain, a helical domain and a C-terminal EH domain. Furthermore, we showed that a switch of the EHD2 N-terminus from the GTPase domain to the membrane controls membrane recruitment and oligomerization. We also demonstrated that EHD2 localizes to caveolae leading to their stabilization at the plasma membrane.
More recently, we determined the crystal structure of an EHD4 construct in a membrane-binding competent, oligomerized form. Activation of EHDs is accompanied by a large-scale conformational movement of the helical domain. This movement aligns the membrane binding sites in the helical domains with the lipid surface. We demonstrated that a series of loop rearrangements accompanies oligomerization. Based on these results, we proposed a molecular model of how EHDs are recruited to membranes to form a membrane-remodeling scaffold (Figure 1)
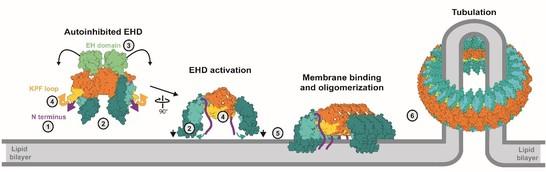
Figure 1: Activation model of EHDs. Upon membrane recruitment, the N-terminus is released into the membrane (1). The helical domains rotate to allow membrane binding (2) which may displace the EH domain (3). The KPF loop moves into the empty GTPase domain pocket (4), therefore creating a new oligomerization interface (5). The assembly into ring-like oligomers may occur via an additional interface in the GTPase domain (Melo et al., 2017).
In addition, we studied the physiological function of EHD2 by characterizing an EHD2 knockout mouse model. Using a variety of different techniques, we discovered a role of EHD2 in regulating caveolae- and CD36-dependent fatty acid uptake (Figure 2).

Figure 2: EHD2 controls cellular lipid uptake. A) EHD2 KO animals are viable and born at normal Mendelian ratio but display increased gonadal fat deposits (B) and increased fat amounts surrounding the heart (C). However, neither body (not shown) nor heart weight (D) are significantly changed. E) FACS-based lipid uptake assays of fluorescent fatty acids in differentiated adipocytes show increased fatty acid uptake in the absence of EHD2. F) Un-treated pre-adipocytes or differentiated adiopocytes display increased LDs, as shown by BODIPY staining. G) EHDdel/del brown adipocytes show an increased number of caveolae that are detached from the plasma membrane (compare arrowheads).
In the next funding period, we will explore the structural basis of how EHDs perform their function at membranes. To this end, we will study structures of membrane-bound assemblies of EHDs with and without interaction partners using cryo-electron tomography, followed by a structure-function approach with biochemical and cell-based assays. In this way, we will elucidate the molecular architecture of membrane-bound EHD scaffolds and their interaction partners as a prerequisite to understand their cellular function in membrane trafficking.
Associated staff: Dr. Arthur Melo (until 2018), Dr. Claudia Matthäus, Dr. Thiemo Sprink, Stephan Grunwald, Elena Vazquez Sarandeses, Saif Mohd
References:
- Okada, A.K., Teranishi, K., Ambroso, M.R., Isas, J.M., Vazquez-Sarandeses, E., Lee, J.Y., Melo, A.A., Pandey, P., Merken, D., Berndt, L., Lammers, M., Daumke, O., Chang, K., Haworth, I.S., and Langen, R. Lysine acetylation regulates the interaction between proteins and membranes. Nat Commun 12, 6466 (2021)
- Wilhelmi, I., Grunwald, S., Gimber, N., Popp, O., Dittmar, G., Arumughan, A., Wanker, E.E., Laeger, T., Schmoranzer, J., Daumke, O., and Schürmann, A. The ARFRP1-dependent Golgi scaffolding protein GOPC is required for insulin secretion from pancreatic β-cells. Mol Metab 45, 101151 (2021)
- Grunwald, S., Hopf, L.V.M., Bock-Bierbaum, T., Lally, C.C.M., Spahn, C.M.T., and Daumke, O. Divergent architecture of the heterotrimeric NatC complex explains N-terminal acetylation of cognate substrates. Nat Commun 11, 5506 (2020)
- Matthaeus, C., Lahmann, I., Kunz, S., Jonas, W., Melo, A.A., Lehmann, M., Larsson, E., Lundmark, R., Kern, M., Bluher, M., Olschowski, H., Kompa, J., Brugger, B., Muller, D.N., Haucke, V., Schurmann, A., Birchmeier, C., and Daumke, O. EHD2-mediated restriction of caveolar dynamics regulates cellular fatty acid uptake. Proc Natl Acad Sci U S A 117, 7471-7481 (2020)
- Xavier, A., Al-Zeer, M.A., Meyer, T.F., and Daumke, O. hGBP1 Coordinates Chlamydia Restriction and Inflammasome Activation through Sequential GTP Hydrolysis. Cell Rep 31, 107667 (2020)
- Faelber, K., Dietrich, L., Noel, J.K., Wollweber, F., Pfitzner, A.K., Muhleip, A., Sanchez, R., Kudryashev, M., Chiaruttini, N., Lilie, H., Schlegel, J., Rosenbaum, E., Hessenberger, M., Matthaeus, C., Kunz, S., von der Malsburg, A., Noe, F., Roux, A., van der Laan, M., Kuhlbrandt, W., and Daumke, O. Structure and assembly of the mitochondrial membrane remodelling GTPase Mgm1. Nature 571, 429-433 (2019)
- Matthäus C., Lahmann, I., Kunz S., Jonas, W., Melo, A.A., Lehmann, L., Larsson, E., Lundmark, R., Haucke, V., Müller, D.N., Schürmann, A., Birchmeier, C., Daumke O. EHD2-mediated restriction of caveolar dynamics regulates cellular lipid uptake. BioRxiv 511709 (2019)
- Melo, A.A., Hegde B.G., Shah C., Larsson E., Isas J.M., Kunz S., Lundmark R., Langen R. & Daumke, O. Structural insights into the activation mechanism of dynamin-like EHD ATPases. Proc Natl Acad Sci.114, 5629-5634 (2017)
- Daumke, O. & Praefcke, G.J.K. Mechanisms of GTP hydrolysis and conformational transitions in the dynamin superfamily. Biopolymers 105, 580-593 (2016) (Review)
- Dick, A., Graf L., Olal D., von der Malsburg, A., Gao S., Kochs, G. & Daumke, O. Role of nucleotide binding and GTPase domain dimerization in dynamin-like myxovirus resistance protein A for GTPase activation and antiviral activity. J. Biol. Chem. 290, 12779-12792 (2015)
- Senju, Y., Rosenbaum, E., Shah, C., Hamada-Nakahara, S., Itoh, Y., Yamamoto, K., Hanawa-Suetsugu, K., Daumke, O. & Suetsugu, S. Phosphorylation of PACSIN2 by protein kinase C triggers the removal of caveolae from the plasma membrane.
- J Cell Sci. 128, 2766-2780 (2015)
- Shah, C., Hegde, B.G., Moren, B., Behrmann, E., Mielke, T., Moenke, G., Spahn, C.M., Lundmark, R., Daumke, O. & Langen, R. Structural insights into membrane interaction and caveolar targeting of dynamin-like EHD2. Structure 22, 409-420 (2014)
- Moren, B., Shah, C., Howes, M.T., Schieber, N.L., McMahon, H.T., Parton, R.G., Daumke, O. & Lundmark, R. EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization. Mol. Biol. Cell. 23, 1316-1329 (2012)
- Faelber K., Held M., Gao S., Posor Y., Haucke V., Noe F., and Daumke O. Structural insights into dynamin-mediated membrane fission. Structure 20, 1621-28 (2012) (Review)
- Brand, J., Smith, E.S., Schwefel, D., Lapatsina, L., Poole, K., Omerbašić, D., Kozlenkov, A., Behlke, J., Lewin, G.R., Daumke, O. A stomatin dimer modulates the activity of acid-sensing ion channels. EMBO J 31, 363546-46 (2012)
- Daumke, O., Lundmark, R., Vallis, Y., Martens, S., Butler, P.J. & McMahon, H.T. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature 449, 923-927 (2007)