A01 - Structural and functional organization fo endocytic scaffolds within the periactive zone
Principal Investigators:
Prof. Dr. Volker Haucke, FMP Berlin
Dr. Tanja Maritzen, FMP Berlin
Communication within the nervous system involves the exocytic fusion of neurotransmitter-filled synaptic vesicles (SVs), neurosecretory organelles that comprise distinct sets of proteins and lipids, at active zone membranes. Following exocytic fusion SVs are recycled locally (Figure 1, Haucke et al., 2011) largely via clathrin-mediated endocytosis (CME) of SV proteins within the surrounding periactive zone. Clathrin-based SV recycling in addition to clathrin (von Kleist et al., 2011) involves endocytic adaptors such as stonin 2 (Diril et al., 2006) and AP180 (Koo et al., 2011) as well as accessory proteins that contribute to SV cargo sorting, membrane deformation, remodelling, and fission. Although substantial progress has been made towards unravelling the mechanics of CME, the spatio-temporal dynamics of endocytic proteins within the endocytic or periactive zone and the underlying molecular mechanisms have remained elusive. Such spatio-temporal control of the SV cycle within the nerve terminal likely involves membrane-associated multidomain scaffolds including intersectin (Figure 2, Pechstein at al., 2010) and Eps15.
Within project A01 we investigate the role of Eps15/intersectin as a molecular scaffold linking SV sorting adaptors such as stonin 2/stoned B to periactive zone organization. We make combined use of cell biological, biochemical, and genetic approaches, paired with electron and super-resolution light microscopy (SRLM) techniques to unravel the structural and functional organization of the Eps15/ intersectin-based endocytic scaffold at synapses. On the long run we expect to gain new insights into the role of endocytic scaffolds in presynaptic organization and exo-endocytic coupling.
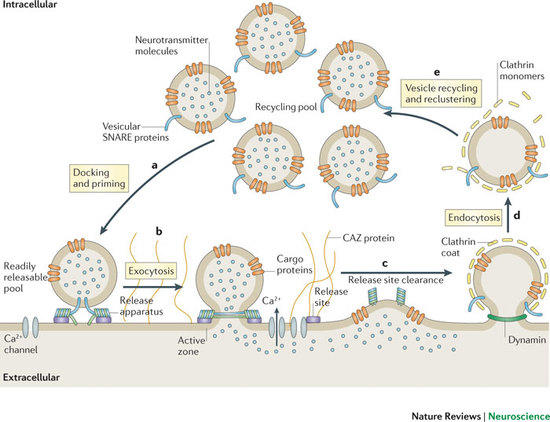
Figure 1: Schematic depiction of the exo-endocytic cycle of SVs. The AZ is a specialized matrix for exocytosis, whereas SV endocytosis preferentially occurs at the surrounding periactive zone. SV proteins other than synaptobrevin are summarized as "cargo proteins" (taken from Haucke et al., Nat Rev Neurosci 2011).
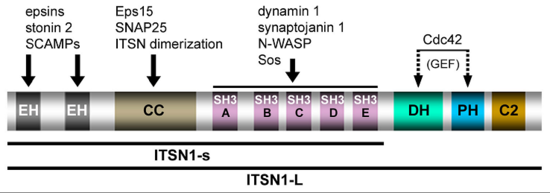
Figure 2: Domain structure and protein interactions of intersectin 1L (ITSN1-L) (taken from Pechstein et al., Biochem. Soc. Trans. 2010).
References:
-
Haucke, V., Neher, E., Sigrist, S.J. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci. 12, 125-136 (2011)
-
von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Kräusslich HG, Shupliakov O, Robinson PJ, McCluskey A, Haucke V. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell, 146, 471-484 (2011)
-
Koo, S.Y., Markovic, S., Puchkov, D., Mahrenholz, C., Beceren-Braun, F., Maritzen, T., Dernedde, J., Volkmer, R., Oschkinat, O., Haucke, V. SNARE motif-mediated sorting of synaptobrevin by the endocytic adaptors CALM and AP180 at synapses. Proc. Natl. Acad. Sci. USA, 108, [advance online publication] (2011)
-
Pechstein, A., Bacetic, J., Vahedi-Faridi, A., Gromova, K., Sundborger, A., Tomlin, N., Krainer, N., Vorontsova, O., Schäfer, J.G., Owe, S.G., Cousin, M.A., Saenger, W., Shupliakov, O., and Haucke, V. Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2. Proc. Natl. Acad. Sci. USA, 107, 4206-4211 (2010)
- Diril, M. K., Wienisch, M., Jung, N., Klingauf, J., and Haucke, V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization. Dev. Cell 10, 233-244 (2006)